Expanding
our knowledge
by working
with patients
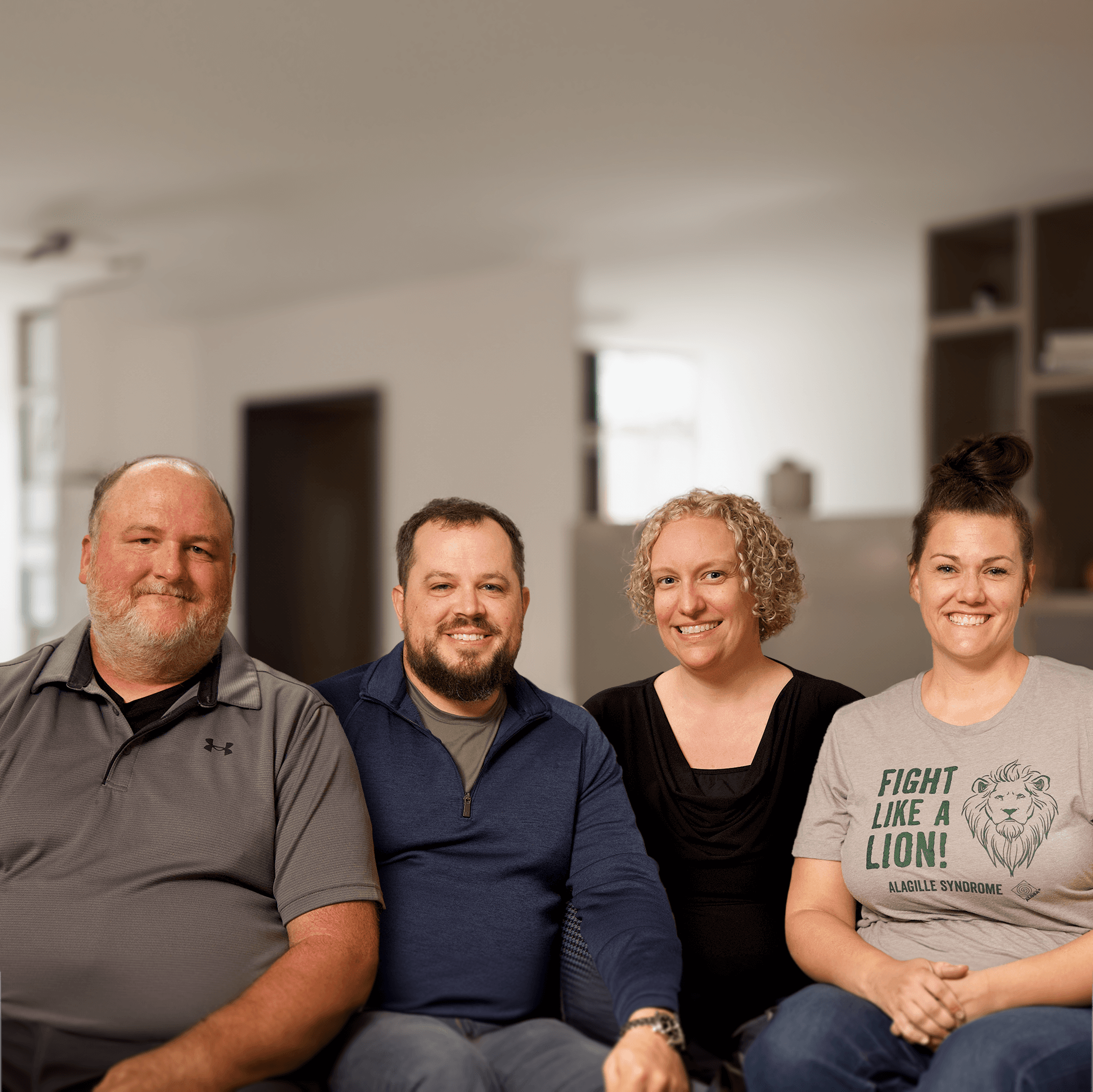
At Mirum, we’re not just working for people with rare disease—we’re working with them, committed to doing what we can to improve their lives. We are immersed in the community, connecting with advocates and listening to patients and their families to truly understand the burdens they face. These insights lend the perspective needed to help improve and extend their lives in meaningful ways.
We’re constantly amazed and inspired by these remarkable and resilient families.
Here are just some of their stories:
We’re constantly amazed and inspired by these remarkable and resilient families.
Here are just some of their stories:


Featured Story:
The PFIC Experience
Hear from the parents of a child with progressive familial intrahepatic cholestasis share how the disease has impacted their lives and the lives of their loved ones.



Meet Cedar, a Pediatric Patient with Progressive Familial Intrahepatic Cholestasis (PFIC)
If you’d like to learn more about the above
conditions or other rare diseases,
check out our resources.
SEE AVAILABLE RESOURCES